BOTOX: FDA Approved for Crow’s Feet. Tansavatdi Facial Plastic Surgery 2835 Townsgate Road, Suite 100, Westlake Village, CA 91361 (805) 715-4996
BOTOX® – FDA Approved for Crow’s Feet
Ophthalmologists have been using BOTOX® to treat blepharospasm (eye twitching) for decades. In 2002, the Federal Drug Administration (FDA) approved the use of BOTOX® for specific treatments within the cosmetic industry. Initially, BOTOX® was used for temporarily diminishing facial frown lines and the wrinkles that are located between the eyebrows. The FDA has now approved the use of BOTOX® to diminish crow’s feet as well.
In 2013, BOTOX® Cosmetic received approval from the FDA for use in diminishing lateral canthal lines, which are also referred to as crow’s feet. Crow’s feet appear due to the repeated use of the muscles located on the outer corners of the eyes.
Facial Expressions Responsible for the Appearance of Crow’s Feet
Common facial expressions that use the muscles located on the outer corners of your eyes, where crow’s feet appear, include:
- Smiling
- Squinting
- Frowning
Dr. Kristina Tansavatdi Specializes in Using BOTOX® Cosmetic
Today, Dr. Kristina Tansavatdi can address crow’s feet, frown lines and the wrinkles between your eyebrows using the FDA approved purified neurotoxin BOTOX® Cosmetic.
BOTOX® Cosmetic is Made from Botulinum Neurotoxin
BOTOX® Cosmetic is a product made from the botulinum neurotoxin and is the only drug approved by the FDA for the treatment of crow’s feet.
Two Clinical Efficacy and Safety Studies Used by FDA to Approve BOTOX® Cosmetic
In its original state, botulinum neurotoxin has the ability to cause a serious disease leading to muscle paralysis throughout the body; however, in its purified state, it can be used to target the muscles that cause crow’s feet, reducing the signs of aging.
A total of 833 adults participated in these studies. These adults displayed moderate to severe crow’s feet. Each participant was randomly assigned an injection of either an inactive placebo or the BOTOX® Cosmetic. Results indicate that individuals treated with BOTOX® Cosmetic injections had less visible crow’s feet than those participants who were administered placebo injections.
Related:
How BOTOX® Cosmetic Works
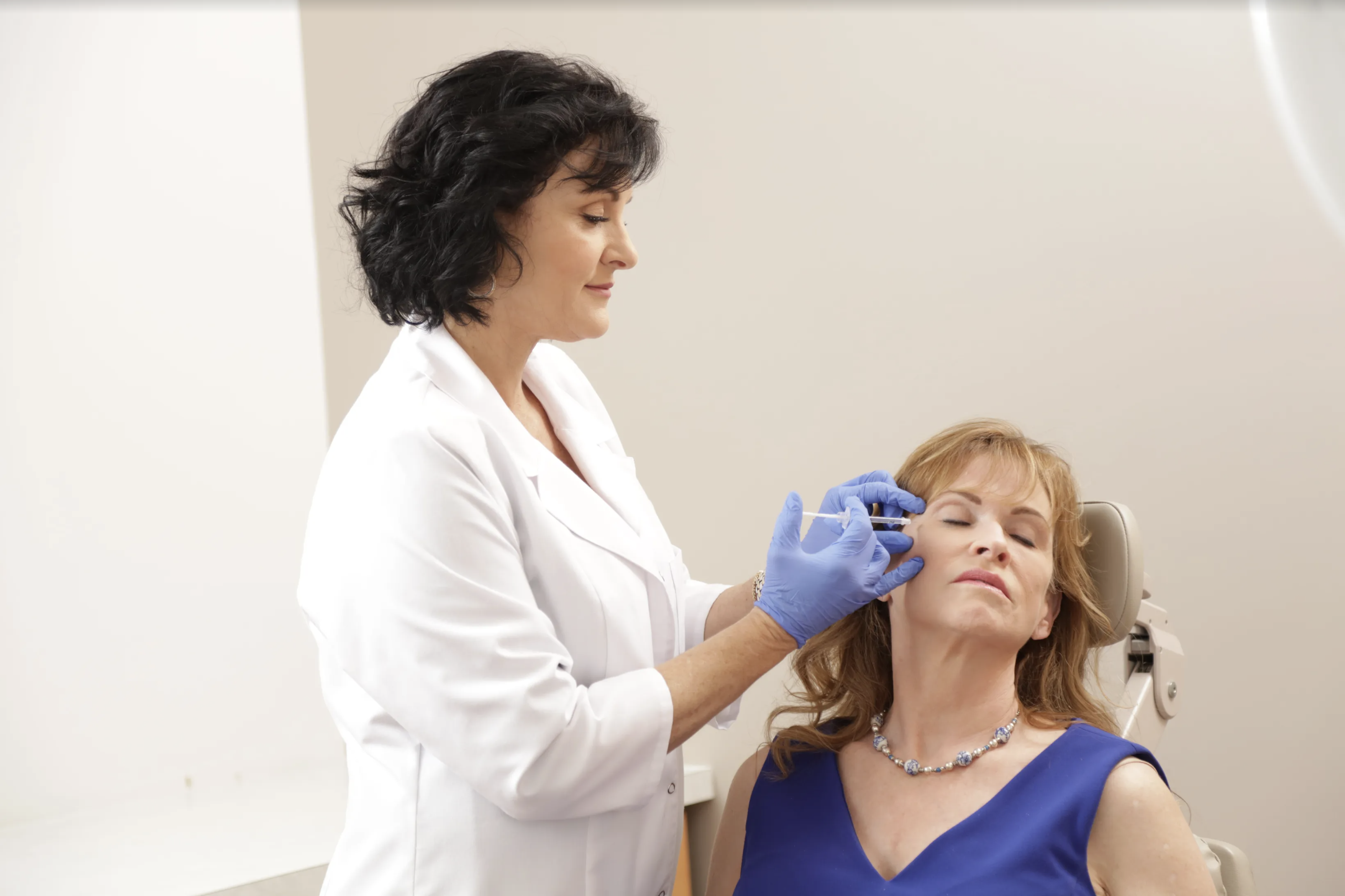
When Dr. Tansavatdi injects the FDA approved BOTOX® Cosmetic directly into the muscles that cause crow’s feet, the muscle is temporarily paralyzed. This, in turn, prohibits the muscles from tightening. Because these muscles are now relaxed, they will no longer tighten; thus, the crow’s feet become less visible.
What to Expect During Your Treatment for Crow’s Feet
Diminishing crow’s feet using BOTOX® Cosmetic offers patients a natural looking solution to the lines next to their eyes. Treatment results are visible within the first three to five days. After the BOTOX® Cosmetic treatment, most of Dr. Tansavatdi’s patients state that they feel as if their eyes appear more open and that they look more rested.
The Results
The results of a BOTOX® Cosmetic treatment for crow’s feet usually lasts anywhere from three to six months. At which time, you can return to Dr. Tansavatdi for another treatment. Some patients find that their results last longer with repeated injections. BOTOX® Cosmetic can even be combined with other types of facial rejuvenation procedures including laser skin resurfacing.
If you live in or near Thousand Oaks or Westlake Village and you are interested in receiving BOTOX® treatments, contact Dr. Tansavatdi today. She specializes in treating crow’s feet, frown lines and the wrinkles between the eyebrows using the FDA approved drug BOTOX® Cosmetic.

BOTOX: FDA Approved for Crow’s Feet. Tansavatdi Facial Plastic Surgery 2835 Townsgate Road, Suite 100, Westlake Village, CA 91361 (805) 715-4996